Publicaciones Similares

Biosimilars in Latin America
The regulations for biosimilars vary
considerably from one country to
another, but in general the trend is an
increase in the regulatory standards for their health registration.
Biosimilars in Peru
In Peru, it is necessary to implement criteria regarding the nomenclature of biosimilar medicines and the use of identifiers that allow them to be distinguished from the innovative medicine.

Health System in Peru
The main challenge facing this system is to expand health care for the population that still does not receive basic services.

Biosimilars
It is a biological medicine that contains a
version of the active ingredient of an original biological product (reference product) whose patent has expired.
Biosimilars in Mexico
The regulatory body for the approval of
medicines in Mexico is the Federal Commission for Protection Against Health Risks (COFEPRIS)
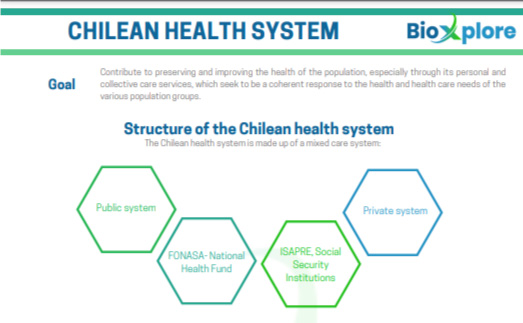
Biosimilars in Chile
The regulatory body in charge of approving
medicines is the National Medicines Agency (ANAMED), which is part of the Public Health Institute of Chile (ISP), under the Ministry of Health.