Publicaciones Similares

Health System in Peru
The main challenge facing this system is to expand health care for the population that still does not receive basic services.

Health System in Argentina
To ensure compliance with area policies for the promotion, preservation, and recovery of the population’s health, strengthening the balance between users, providers, and financiers, in conditions of free
competition, transparency, economic efficiency, and social equity.
Biosimilars in Mexico
The regulatory body for the approval of
medicines in Mexico is the Federal Commission for Protection Against Health Risks (COFEPRIS)
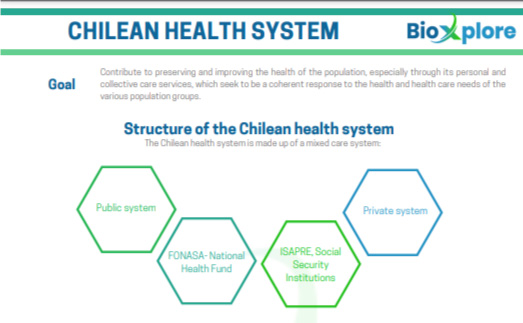
Chilean Health System
Contribute to preserving and improving the health of the population, especially through its personal and collective care services, which seek to be a coherent response to the health and health care needs of the various population groups.

Biosimilars in Brazil
Brazil has designed a set of measures to increase investment in biopharmaceutical research and is promoting the production of national biological drugs.

Colombian Health System
The main objective is to regulate the essential public health service and create conditions of access for the entire resident population of the country, at all levels of care.