Publicaciones Similares

Mexican Health System
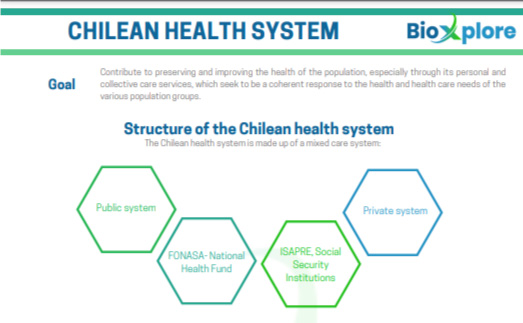
To establish state policies so that the
population has the right to health protection.
Why are prices so high in the US?
An In-depth Analysis of High Prices in the U.S. Delve into the statistics, root causes, and strategic interventions shaping the economic landscape in our comprehensive infographic. Uncover the complexities, explore solutions.

Brazilian Health System
Guarantees universal access to health and finances the system through taxes through the Unified Health System (SUS) and a private sector.

Biosimilars in Chile
The regulatory body in charge of approving
medicines is the National Medicines Agency (ANAMED), which is part of the Public Health Institute of Chile (ISP), under the Ministry of Health.
Why does the Inflation Reduction Act reduce the prices of Pharmaceuticals?
The Inflation Reduction Act (IRA) is used to lower drug prices by imposing extra costs on drug manufacturers that increase their prices above the inflation rate. This forces manufacturers to keep their price increases below the inflation rate to avoid the penalty

Biosimilars in Brazil
Brazil has designed a set of measures to increase investment in biopharmaceutical research and is promoting the production of national biological drugs.