Publicaciones Similares

Biosimilars in Latin America
The regulations for biosimilars vary
considerably from one country to
another, but in general the trend is an
increase in the regulatory standards for their health registration.
Biosimilars in Peru
In Peru, it is necessary to implement criteria regarding the nomenclature of biosimilar medicines and the use of identifiers that allow them to be distinguished from the innovative medicine.

Biosimilars in Chile
The regulatory body in charge of approving
medicines is the National Medicines Agency (ANAMED), which is part of the Public Health Institute of Chile (ISP), under the Ministry of Health.
Consequences of the IRA for the pharma sector
Navigating Pharma’s Evolution: Dive into the consequences of the Inflation Reduction Act 2022. From drug refunds to taxes, Medicare changes, and R&D impacts, the industry is transforming. Stay competitive with our tailored resource solutions. Contact us to thrive amidst evolving pharmaceutical dynamics.

Mexican Health System
To establish state policies so that the
population has the right to health protection.
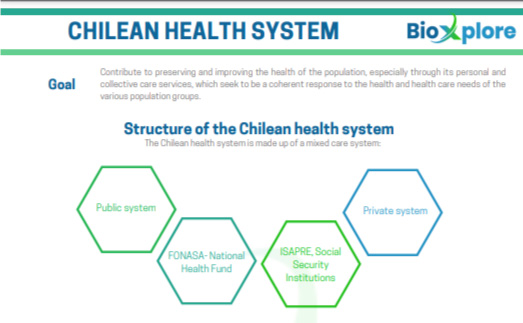
Chilean Health System
Contribute to preserving and improving the health of the population, especially through its personal and collective care services, which seek to be a coherent response to the health and health care needs of the various population groups.